Spontaneous cardiomyopathy -and nephropathy in the owl monkey (Aotus sp.) captivity
Gozalo A. Dagle GE. Montoya E. Weller RE, Málaga CA. Spontaneous cardiomyopathy and nephropathy in the owl monkey (Aotus sp.) in captivity. J Med Primatol 1992:21:279-284.
Clinical and pathologic data were reviewed for 72 owl monkeys that died of Nonhuman Primates, Instituto Veterinario between January 1987 and May 1990 at the Center for Reproduction and Conservation of Nonhuman Primates in Iquitos, Peru. Tissue samples from 39 animals were examined. Hypertrophic cardiac disease (5 1 % of an- imals examined). dilative cardiomyopathy (26%), and nephropathy (87%) were the most common diagnoses. The incidence of all three diseases ap- peared to increase with time in captivity. Nephropathy was less severe in colony-born animals.
A. Gozalo, 1 G.E. Dagle 2
E. Montoya, R.E. Weller 2
C.A. Má1aga 2
Center for Reproduction and Conservation
de Investigaciones Tropicales y de Altura. Universidad Nacional Mayor de San Marcos, Iquitos, Peru: 2Battelle Northwest Laboratories, Biology and Chemistry Department, Richland, WA, U.S.A.
Key words: cardiac disease - nephropathy -night monkey - Cebidae
A. Gozalo, Center for Reproduction and Conservation Nonhuman Primates, instituto Veterinario de Investigaciones Tropicales y de Altura, Universidad Nacional Mayor de San Marcos, Iquitos, Peru.
Accepted for publication May 12, 1992
Introduction
The owl monkey (Aotus species) has been used extensively
for the study of several diseases affecting man [1]. Although owl monkeys have
been reported to die from a variety of illnesses, hemolytic anemia and glomerulonephritis
appear to be the most common causes of mortality in captive populations [3].
Although most of these reports describe the occurrence of glomerulonephritis
and hemolytic anemia in monkeys used for infectious disease research, these
and other spontaneous diseases in the species have also been observed in monkeys
that have h4d no experimental manipulation [1,3,9,10.131, Therefore, in order
to be able to provide proper health care for these monkeys, it is important
that studies be performed to determine the prevalence and etiology of spontaneous
diseases in both wild and captive populations. By doing so, we can more effectively
utilize existing breeding populations and avoid depleting wild populations of
a potentially endangered species. In this report, we describe the occurrence
of spontaneous cardiomyopathy and nephropathy in two species of owl monkey,
Aotus nancymae and Aotus vociferans, that are important causes of death
in our captive breeding populations.
Materials and methods
Clinical and pathologic data were reviewed For 72 owl monkeys that died during a 41-month period (January 1987-May 1990) at the Center for Reproduction and Conservation of Nonhuman Primates (CRCP) in Iquitos, Peru. Sixty-five of the monkeys had been wild-caught in the Peruvian Amazonia and placed in our breeding colonies. while the remaining seven animals were born at the CRCP. All of the animals included in the study were adult animals maintained in captivity for breeding purposes. Husbandry conditions at the CRCP have been previously described [8]. Dead monkeys were refrigerated and necropsies preformed as soon as possible according' to previously described procedures for nonhuman primates [7]. The dia2nosis in each case was based on clinical signs. gross necropsy findings. and histopathological evaluation of tissue specimens. Tissue specimens from 39 selected cases with gross evidence of cardiac or renal disease were collected at necropsy and fixed in 10% neutral buffered formalin. processed routinely. and stained with hematoxylin and eosin. In some cases special staining techniques were employed.
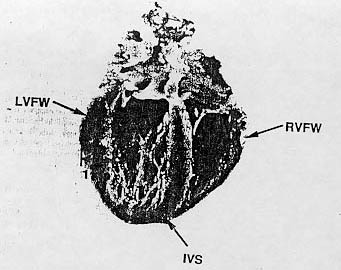
Fig. 1. Owl monkey heart showing marked hypertrophy of the left ventricular free-wall and the interventricular septum. RVFW= right ventricular free-wall: IVS= interventricular septum: LVFW = left ventricular free-wall.
Results
Clinical signs
Clinical signs in animals with cardiomyopathy varied from
muscular weakness to sudden death with- out any previous clinical evidence of
disease. Other signs included, lack of stamina, dyspnea, tachypnea, hepatomegaly,
ascites, and, less frequently, cyanosis, convulsions, and syncope. Those with
advanced nephropathies occasionally presented with, nephrotic syndrome, proteinuria
or hematuria. For urinalyses, specimens were obtained by spontaneous voiding
or manual expression of the bladder. Semi-quantitative assays for protein, glucose,
ketones, bilirubin and occult blood were determined using a reagent-strip test
method [2].
Gross pathology
Common gross necropsy findings included mild to severe concentric hypertrophy of the left ventricle and interventricular septum (Fig. 1). and, less fre- quently dilative cardiomyopathy with pericardial effusion. Both conditions were usually associated with evidence of renal disease. The myocardium.affected animals often contained pale streaks. Pulmonary congestion and edema were the most common abnormalities observed in the lungs. Chronic passive congestion of the liver splenic congestion, and hemorrhagic enteritis were occasionally observed. Subcutaneous edema ascites and hydrothorax was observed in some animals with dilatative cardiomyopathy and nephrotic syndrome. Size of the kidneys varied from animal to animal. In most cases, the renal capsule was slightly to moderately adherent to the capsular surface which varied in texture from smooth to rough and pitted. Randomly scattered gray-white focimeasuring from 0.5 to I mm in diameter were observed on the capsular surface and the cut surface of the renal cortex. In animals with advanced nephropathy, larger foci could be seen on the cut surface of the cortex. and the corticomedullary junction was disrupted and difficult to discern. All other organs and systems appeared normal.
Microscopic pathology
Histopathologic examination of the heart revealed subtle changes in most cases.
In some animals. the myocardium contained only slight areas of focal necrosis
and diffuse interstitial.fibrosis.without inflammation (Fig 2).
The hearts of those monkeys, with chronic dilative cardiomyopathy showed more
severe changes characterized by myocyte atrophy. loss of myofibrils and loss
of the linear array of sarcomeres producing sharp myocyte angulations with increased
interstitial fibrosis (Fig. 3). Contraction band necrosis was observed in one
animal.
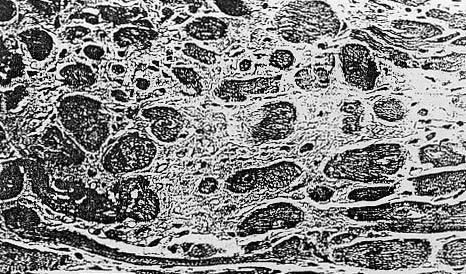
Fig. 2. Photomicrograph demonstrating focal necrosis and diffuse interstial fibrosis of the myocardium. H&E ( X 315).
Those animals with hypertrophic cardiomyopathy exhibited concentric hypertrophy and hyperplasia of myocytes that was largely confined. to the left ventricle. Approximately 87% of the animals examined showed some evidence of chronic nephropa- thy, which included hypercellular glomeruli, dilated renal tubules, and diffuse interstitial fibrosis with mononuclear cell infiltrates. The cellular infiltrates were composed primarily of lymphocytes with occasional plasma cells and eosinophils (Fig. 4).
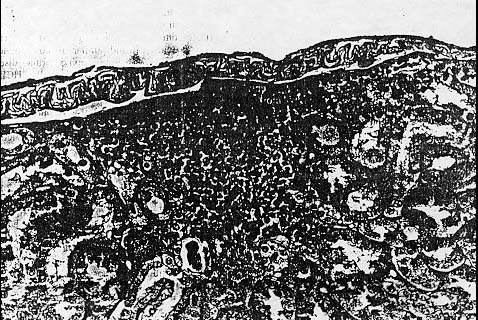
Granular casts were occasionally observed in some of the
dilated tubules along with eosinophilic- staining material that was probably
proteinaceous An origin. Small areas of calcification were also noted in the
medullary portion of the kidney. The glomeruli of those animals in advanced
stages of renal disease were characterized by marked glomerulosclerosis with
adherence of Bowman's capsule, thickening of the wall of the afferent arteriole,
and extensive interstitial and periglomerular fibrosis resulting in distortion
of the capsule (Fig. 5). Hepatic changes were characterized by congestion. slight
lipofuscinosis. diffuse centrilobular to midzonal necrosis. and diffuse vacuolation
of hepatocytes. Congestion and hemorrhage was occasionally observed in the gastrointestinal
tract, along with focal villous atrophy of the small intestine and erosions
in the gastric mucosa.
Discussion
The gross necropsy and histopathological findings observed in the owl monkeys
described in this report are comparable to those previously described for the
species [6], and similar to those found in human subjects with hypertrophic
cardiac disease (HCD) and hypertension [4]. Clinically, HCD is difficult to
diagnose in owl monkeys because of the lack of clinical signs and specific diagnostic
criteria [12]. These animals are often asymptomatic prior to diagnosis or sudden
death, and diagnostic methodologies such as thoracic radiography and electrocardiography
are not reliable in separating animals with and without HCD [12, unpublished
data]. The relationship between blood pressure and HCD is even more difficult
to ascertain since normative values for blood pressure have not been established
for the owl monkey, and reliable methods for measuring blood pressure in awake
or anesthetized animals have not been described. Therefore, we can only speculate,
at this time, about the etiology of the HCD observed in the owl monkeys included
in this study. Although it is possible that hypertension secondary to renal
disease could be the cause of the observed cardiac hypertrophy. other etiologies
such as essential hypertension familial hypertrophic cardiomyopathy. and cardiomyopathic
disease are just as likely. Regardless of etiology, HCD was diagnosed more frequently
(5 1 %) than dilative cardiac disease (26%) in the 39 monkeys selected for histopathological
evaluation (Table 1).
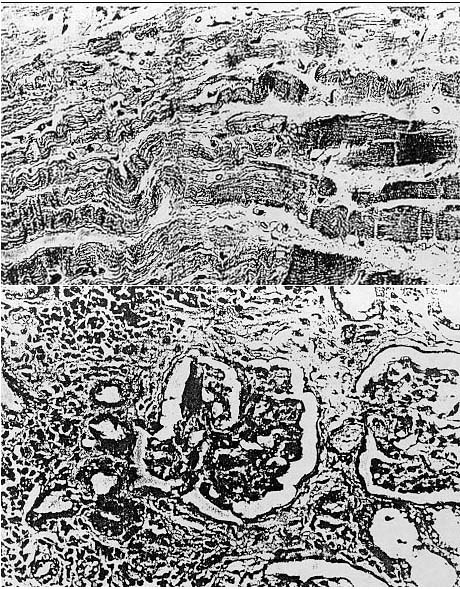
Fig. 5. Photomicrograph showing advanced nephropathy characterized by glomerulosclerosis. thickening of the wall of the afferent arteriole. periglomerular fibrosis, and chronic interstitial inflammation. H&E ( x 250).
Renal disease is one of the more common causes of morbidity
and mortality reported.in the owl monkey, although the etiopathogenesis,
in most cases, remains unknown [1,3,9,101. Infectious diseases, hemoparasites,
endoparasites, environment, and dietary factors have all been suggested as possible
causes of nephropathy in wild-caught and colony- born owl monkeys. In the present
study, diet was the only factor common to all of the animals diagnosed with
HCD and nephropathy. Glomerulonephritis related-to food hypersensitivity has
been reported in man [11], and the composition of the diet fed at the CRCP is
substantially different from that of the natural diet of feral owl monkeys.
The authors believe that the clinical signs exhibited by the owl monkeys included
in this study were the result of chronic cardiac disease, chronic nephropathy,
or a combination of both diseases. It is possible that the observed neuromuscular
signs were partially attributable to hypokalemia or other electrolyte imbalances
resulting from renal disease [5]. Although there was no direct correlation between
the severity of the lesions observed in the heart and kidneys and length of
time in captivity, those animals that died while in quarantine (less than or
equal to four months in captivity) exhibited a lower incidence of HCD and nephropathy
than those monkeys that died after more than six months in captivity (Table
1), suggesting either an age- or captivity-related effect. There was no species
or sex differences with regard to the occurrence or severity of either disease.
Severe nephropathy was only noted in those monkeys held in captivity for more
than twenty months. The four colony-born adult owl monkeys included in this
study showed only slight nephropathy upon histopathological examination of the
kidneys regardless of age (Table 1). This suggests that wild-caught monkeys
are at greater risk for development of significant nephropathy than colony-born
monkeys, possibly due to more frequent exposure to diseases and parasites in
their natural environment. It also suggests that any apparent linkage between
HCD and nephropathy in owl monkeys needs to be further evaluated in prospective
studies where variables such as diet systemic blood pressure. indices of renal
function. and hemoparasites are evaluated to determine what role. if any they
play in the pathogenesis of either disease.
Acknowledgments
Supported by the Peruvian government and the Pan American
Health Organization the Proyecto Peruano de Primatología
"Manuel Moro Sommo" and Battelle Northwest Laboratories.
Table 1. Species, sex, source. and time in captivity of owl monkeys with cardiac and renal disease*
| Species |
Sex
|
Source
|
Time in CaDtivity
|
Gardiomyopathy
|
Nephropathy
|
| A nancymai |
F
|
WC
|
6 days
|
-
|
+
|
| A nancymai |
M
|
WC
|
11 days
|
-
|
+
|
| A nancymai |
F
|
WC
|
20 days
|
HC
|
++
|
| A nancymai |
F
|
WC
|
23 days
|
HC
|
++
|
| A vociferans |
M
|
WC
|
1 month
|
-
|
+
|
| A nancymai |
M
|
WC
|
3 months
|
-
|
+
|
| A nancymai |
M
|
WC
|
3 months
|
-
|
++
|
| A nancymai |
F
|
WC
|
4 months
|
-
|
+
|
| A nancymai |
M
|
WC
|
9 months
|
HC
|
++
|
| A nancymai |
M
|
WC
|
9 months
|
DC
|
++
|
| A nancymai |
M
|
WC
|
10 months
|
HC
|
+
|
| A vociferans |
F
|
CB
|
19 months
|
-
|
+
|
| A vociferans |
F
|
WC
|
19 months
|
HC
|
+
|
| A vociferans |
F
|
WC
|
20 months
|
-
|
+
|
| A vociferans |
F
|
WC
|
20 months
|
DC
|
+++
|
| A nancymai |
M
|
WC
|
21 months
|
HC
|
+++
|
| A vociferans |
M
|
WC
|
27 months
|
DC
|
++
|
| A vociferans |
M
|
WC
|
27 months
|
HC
|
+++
|
| A vociferans |
M
|
WC
|
32 months
|
-
|
++
|
| A vociferans |
F
|
WC
|
32 months
|
HC
|
++
|
| A vociferans |
F
|
WC
|
34 months
|
DC
|
++
|
| A nancymai |
F
|
WC
|
35 months
|
H C
|
+++
|
| A vociferans |
M
|
WC
|
39 months
|
DC
|
++
|
| A vociferans |
M
|
WC
|
40 months
|
DC
|
+++
|
| A vociferans |
F
|
WC
|
42 months
|
HC
|
++
|
| A vociferans |
F
|
WC
|
42 months
|
DC
|
++
|
| A vociferans |
F
|
CB
|
45 months
|
HC
|
+
|
| A vociferans |
F
|
WC
|
45 months
|
HC
|
+++
|
| A nancymai |
M
|
WC
|
49 months
|
HC
|
+
|
| A vociferans |
F
|
WC
|
56 months
|
DC
|
++
|
| A vociferans |
F
|
CB
|
59 months
|
HC
|
+
|
| A vociferans |
M
|
WC
|
61 months
|
HC
|
+
|
| A vociferans |
M
|
WC
|
61 months
|
DC
|
+++
|
| A vociferans |
M
|
WC
|
63 months
|
HC
|
+
|
| A nancymai |
M
|
CB
|
91 months
|
HC
|
+
|
| A nancymai |
M
|
WC
|
96 months
|
DC
|
++
|
| A nancymai |
F
|
WC
|
99 months
|
HC
|
++
|
| A nancymai |
F
|
WC
|
100 months
|
HC
|
++
|
| A nancymai |
F
|
WC
|
104 months
|
HC
|
+++
|
*WC=wild-caught; CB=colony-born; HC=hypertrophic cardiomyopathy; DC=dilative cardiomyopathy; Severity grade: + = slight; + + = moderate; +++ = severe.
References
| 1. AIKAWA M. BRODERSON JR, IGARASHI I, JACOBS G, PAPPAIOANOU M, COLLINS, WE, CAMPBELL, CC: "An Atlas of Renal Disease in Aotus monkeys, with Experimental Plasmodial Infection." Arlington: American Institute of Biological Sciences, 1988. |
| 2. BROBST DF: Urinalysis and associated laboratory procedures. Vet Clin North Am [Small Anim Pract] 19:929- 949, 1989. |
| 3. CHALIFOUX LV, BRONSON RT, SEHGAL P, BLAKE BJ, |
| KING NW: Nephritis and hemolytic anemia in owl monkeys (Aotus trivirgatus). Vet Pathol 18:23-37, 1981. |
| 4 COTRAN RS, KUMAR V, ROBBINS SL: "Robbins Pathological Basis of Disease." 4th ed. Philadelphia, W.B. Saunders, 1989. |
| 5. FOURNIER A, LAGRUE G: Les hypentensions, avec aldosteronisme primaire; Physiopathologie, diagnostic et indications therapeuthiques. Coeur Med Interne 10:571-590. 1971. |
| 6. GIBSON SV, BESCH-WILLIFORD CL, SKAVLEN PA, STILLS HF, KELLEY ST, GREEN TJ: Cardiovascular lesions in captive owl monkeys (Aotus species). Lab Anim Sci 34:500, 1984. |
| 7. Goss L: Necropsy procedure for wild animals. In Jones, TC, Gleiser, CA (eds.). "Veterinary Necropsy Procedures." Philadelphia. J.B. Lippincott Co.,1954. |
| 8. GOZALO A. MONTOYA E: Reproduction of the owl monkey (Aotus nancymai) (Primates: Cebidae) in captivity. Am J Primatol 21:61-68, 1990. |
| 9. HUNT RD, VAN ZWIETEN MJ , BAGGS RB, SEHGAL PK, KING NW, ROACH SM, BLAKE BJ: Glomerulonephritis in the owl monkey (Aotus trivirgatus). Lab Anim Sci 26:1088-1092, 1976. |
| 10. KING NW, BAGGS RB, HUNT RD, VAN ZWIETEN MJ, MACKEY JJ: Glomerulonephritis in the owl monkey (Aotus trivirgatus): ultrastructural observations. Lab Anim Sci 26:1093- 1103, 1976. |
| 11. McCRORY WW, BECKER CG, CUNNINGHAM-RUNDLES C. KLEIN RF, MOURADIAN J, REISMAN L: Immune complex glomerulonephropathy in a child with food hypersensitivitv. Kidney Int 30:592-598, 1986. |
| 12. RODGER RF, HARTLEY LH, RINGLER DJ, Nicolosi RJ: Hypertrophic cardiomyopathy in owl monkeys (Aotus trivirgatus): clinical diagnosis and clinicopathologic correlations. Lab Anim Sci 36:561, 1986. |
| 13. VOLLER. A, DAVIES, DR, HUTT, MSR: Quartan malarial infections in Aotus trivirgatus with special reference torenal pathology. Br J Exp Pathol 54:457-468. 1973. |
