

A.) Saimiri sciureus Linnaeus, 1758
1. Common name; local names
Squirrel monkey; Fraile, frailecito, mono ardilla, huasa,
pichico.
2. External characters (Fig. 8)
 |
Pelage with fine, short and dense hair. Crown, parietal and occipital region grey, forehead with a broad, arching white band above the eyes. Muzzle blackish. Sides of the neck with whitish, plushy hair. Upperparts, upper arm and thigh grey, forearm and lower leg reddish-orange. Underparts creamy-yellow. Tail grey with a black terminal tuft. Further information is provided by HERSHKOVITZ (1984). |
3. General information
With a total length of up to 80 cm and weight up to 1000
g or somewhat more in the case of adult males, this species belongs to the small
primates. Squirrel monkeys live preferentially in primary, secondary and remnant
forests of the bosque de colina type. Travelling, foraging and resting
take place in the lower and middle layers of the forest. They sleep in trees
and bushes with dense tangles of vines and at the base of the crown of palms.
Social groups comprise 20-80 individuals. The social organization is polygamous,
every group contains several adults of both sexes. However, the number of dominant,
reproductively active males does not exceed 10 individuals per group. A peculiar
characteristic of this species is the accumulation of adipose tissue in adult
males previous to the mating season in July/August ("fatted male phenomenon";
DUMOND & HUTCHINSON, 1968). The birth season comprises the period between
December and February. One infant is born per birth and is carried and cared
for mainly by the mother. Squirrel monkeys are generally found in interspecific
association with Cebus apella and Lagothrix lagotricha, but they
have also been observed in association with Cacajao calvus ucayalii. The
diet is composed of insects and fruits. They frequently invade plantations of
com and bananas, and therefore, S. sciureus as well as S. boliviensis are
considered as agricultural pests in some part of Amazonia. Further information
about ecology and behaviour is provided by BALDWIN & BALDWIN (198 1), ENCARNACIÓN
(1990), and THORINGTON (1968).
4. Status
The numerous censuses carried out between 1976 and 1987 indicate that S. sciureus is abundant in Peru. Its status is therefore considered as COMMON.
5. Distribution, conservation and protection (Map 5)
S. sciureus has a wide distribution in Peru. According to HERSHKOVITZ (1977) it is found in the Departments of Loreto, Amazonas and San Martín. In Loreto it is found north of the rivers Marañón and Amazonas to the border with Colombia and Ecuador, and from the tight bank of the Ucayali to the Yavarí in the east. The southern limit is the Rio Abujao where this species is found in sympatry with S. boliviensis. During explorations in 1988, both species were found at the Quebrada Carahuaite, a point further north of the Rio Tapiche (J. TAPIA, personal communication), which indicates that the area of sympatry is even greater.
S. sciureus is represented in Peru by the subspecies S. s. macrodon, which is found in the Comunal Reserve Tamshiyacu-Tahuayo.
B.) Saimiri boliviensis E. Geoffroy & R. de Blainville, 1834
1. Common name; local names
Squirrel monkey; Fraile, frailecillo, mono ardilla, huasa, pichico.
2. External characters
Pelage
in general similar to S. sciureus but forehead and crown between greyish-agouti
to entirely black without broad white band above eyes, superciliary region with
few agouti or whitish hair; supraorbital vibrissae inconspicuous. Slender tail,
terminal blackish portion not tufted. This species is represented in Peru by
two subspecies: S. b. peruviensis and S. b. boliviensis. In S.
b. peruviensis crown and preauricular patch greyish-agouti in the male
and predominantly blackish in the female; in S. b. boliviensis crown
entirely blackish in both sexes. Further information is provided by HERSHKOVITZ
(4984).
3. General information
Length and weight are similar to S. sciureus. It lives preferentially in seasonally inundated primary, secondary and remnant forests. Groups comprise 100 individuals or more. Interspecific associations with Cebus apella or Cebus albifrons are frequently observed. Ecology and behaviour are similar to S. sciureus. Further information is provided by BOINSKI & MITCHELL (1992), ENCARNACIÓN (1990), MITCHELL (1990), MITCHELL et al. (1991), PODOLSKY (1990), SOINI (1986), and TERBORGH (1983, 1985).
4. Status
S. boliviensis is a common species of Peruvian Amazonia. It is hunted for "sanitary" reasons in areas with large cultivations of corn and legumes. Its status is considered as COMMON.
5. Distribution, conservation and protection (Map 5)
S. boliviensis is represented by two subspecies: S. b. peruviensis and S. b. boliviensis. According to HERSHKOVITZ (1984), S. b. peruviensis is distributed south of the rivers Marañón and Amazon between the rivers Ucayali and Huallaga, and east of the Ucayali in sympatry with Saimiri sciureus macrodon from about 04º00ºS southward to the Rio Abujao. The subspecies S. b. boliviensis is distributed in the southeast of Peru, from the Rio Abujao in the Department of Ucayali to the Bolivian border in the south. It is also found in parts of the Departments of Huánuco, Pasco, Junín, and Cusco. S. b. peruviensis is represented in the National Reserve of Pacaya-Samiria, and S. b. boliviensis in the National Parks of Manú and Yanachaga-Chemillén.
C.) Aotus vociferans Spix, 1823
1. Common name; local names Night monkey; Mono nocturno, musmuqui, buri buri.
2. External characters
Pelage in general greyish to buffy greyish with dense, short and velvety hair. Crescent-shaped creamy-white patch above each eye, delimited by dark medial and lateral bands. These bands converge diffusely on the base of the crown, forming a whirl. Upperparts and outside of limbs varying between greyish and buffy-greyish. Underparts except throat and inner side of upper arm and thigh huffy, forearm and lower leg buffy-grey, throat grey. Proximal part of tail buffy, distal part blackish. Further information is provided by HERSHKOVITZ (1983).
3. General information
Members of the genus Aotus are the only nocturnal species
among the neotropical primates. Until 1983 the genus was represented by the
species A. trivirgatus only. In a revision of the genus, HERSHKOVITZ (1983)
considered nine species which were grouped into two species group, the "grey-neck
group" and the "red-neck group".
A. vociferans belongs to the "grey-neck group" and reaches a total length of 67 cm and a weight of 1000 g. It lives preferentially in the lower parts (bajiales) of primary, secondary and remnant forests, but it is also be found in bosque de colina-type forests and in riverine vegetation along rivers and creeks. Travelling, foraging and resting take place in the lower and middle layers of the forest. During the day it sleeps exclusively in hollows located between 15 and 30 m height in mature, senescent or dead trees. Groups formed by an adult pair and their offspring of varying age generally comprise 2-4, exceptionally 5 individuals. They do not have a well-defined birth season, but most births occur,between October and January. One infant is born per birth and is mainly carried and cared for by the father. The diet consist basically of fruits, including several species of the genus Ficus (Moraceae), but insects and flowers are consumed, too. Further information about ecology and social organization are provided by AQUINO & ENCARNACIÓN (I 986a, 1988) and AQUINO et al. (1990).
4. Status
The numerous censuses carried out by AQUINO & ENCARNACIÓN (1988) indicate that this species is relatively abundant. Hunting of the species is restricted to the vicinity of large human settlements. The status is considered as COMMON.
A. vociferans is the only representative of the "grey-neck group" in Peru. During explorations on the upper Rio Napo in 1982 and 1983 and later in the basin of the Rio Nanay, groups were captured that differed phenotypically and genotypically from the typical A. vociferans, which might represent a different, but sympatric species.
5. Distribution, conservation and protection (Map 6)
According to HERSHKOVITZ (1983) and AQUINO & ENCARNACIÓN (I 988),A. vociferans is distributed in northeastern Peru between the rivers Marañón and Amazon in the south and Putumayo in the north. In the west the distribution extends to the Rio Chinchipe. The species is missing from an enclave between the rivers Tigre and Pastaza where A. nancymae is found. A. vociferans is not represented in any of the protected areas of Peru. Therefore, a protected area should be established within the distributional range of this species.
D.) Aotus nancymae* Hershkovitz, 1983
* Reasons for changing nancymae into nancymae are explained in AQUINO et al. (1990).
1. Common name; local names Night monkey; Musmuqui, mono nocturno, mono lechuza.
2. External characters (Plate2)
 |
Pelage generally greyish-agouti with dense, short and velvety hair. Whitish crescent-shaped patches above each eye delimited by black medial and lateral bands that do not converge at the base of the crown. Upperparts and outside of limbs greyish-agouti; medial, slightly blackish and not very well-defined band from the neck to the base of the tail. |
Underparts (including throat) and inner side of upper arm and thigh reddish-orange, forearm and lower leg greyish-agouti. Proximal portion of tail dark-reddish, distal portion blackish. Further information is provided by HERSHKOVITZ (1983).
3. General information
With a total length of 74 cm and a weight of 650- 1000 g,
A. nancymae belongs to the small primates. It lives preferentially in
bajiales of primary, secondary or remnant forests, but can also be found
in bosque de colina. Travelling, foraging, and resting take place in
the lower and middle layers of the forest. Occasionally, they come down to the
ground to reach isolated fruit trees. During the day it sleeps in hollows and
caves of trunks of senescent or dry trees, but makes also use of tangles of
vines and agglomerations of epiphytes, hemiepiphytes and hemiparasites. Sleeping
sites are generally located between 7 and 16 m height. Groups formed by an adult
pair and their offspring of varying age include 2-5, exceptionally 6 individuals.
The diet consists basically of fruits, but nectar and insects are also consumed.
Further information about ecology and social organization is provided by AQUINO
& ENCARNACIÓN (1986a, 1986b), AQUINO et al. (1990), and HERSHKOVITZ
(1983).
4. Status
Results of numerous censuses indicate that this species is relatively abundant. Hunting of this species is restricted to the vicinity of large settlements. Its meat is not well-liked, probably due to the presence of a subcaudal gland that produces a secretion with an unpleasant odour. The species is considered as COMMON.
5. Distribution, conservation and protection (Map 6)
According to HERSHKOVITZ (1983) and AQUINO & ENCARNACIÓN (1988), A. nancymae is distributed in northeastern Peru south of the rivers Maraft6a and Amazon to about,07º00ºS, and from the Eastern Cordillera in the west to the Rio Yavarí in the east. It is also found in an enclave between the rivers Tigre and Pastaza north of the Marañón to about 03º00ºS. This species is represented in the Pacaya-Samiria National Reserve and in the Comunal Reserve Tamshiyacu-Tahuayo.
E.) Aotus nigriceps Dollman, 1938
1. Common name; local names Night monkey; Musmuqui, mono lechuza.
2. External characters
Pelage generally buffy-grey with dense, short and velvety hair. Whitish crescent-shaped patches above each eye delimited by black medial and lateral bands that do not converge at the base of the crown. Upperparts and outside of limbs buffy-grey. Chin, throat, lateral part of neck, underparts, inner side of arm up to wrist and inner side of leg up to ankle pale-orange. Proximal portion of tail reddish-orange, distal portion brownish. Further information is provided by HERSHKOVITZ (1983).
3. General information
A. nigriceps belongs to the "red-neck group". Habitat preference, social organization and other aspects of its ecology are similar to the other species of this group and are described by WRIGHT (1979, 1985).
4. Status
Like other species of the genus, A. nigriceps is hunted
in the vicinities of large settlements. Studies by WRIGHT (1979, 1985) in Puerto
Berm6dez (Department of Huánuco) and observations by Aquino (unpublished
data) in Puerto Inca (Department of Huánuco), Abijao and Inuya (Department
of Ucayali), and Tahuamanú (Department of Madre de Dios) indicate a relative
abundance. However, since no information is available about population densities
in other areas, we consider its status as INDETERMINATE.
5. Distribution, conservation and protection (Map 6)
According to HERSHKOVITZ (1983), A. nigriceps is distributed from approximately the Rio Cushabatay (07º00ºS) southward to the Rio Madre de Dios, and from the Rio Huallaga in the west to the Brazilian border in the east. A. nigriceps is represented in the Manú National Park.
F.) Aotus miconax Thomas, 1928
1. Common name; local names Night monkey; Musmuqui, tutamono, mono lechuza.
2. External characters
Pelage generally brownish-agouti to greyish, with long, dense and velvety hair. White crescent-shaped patches above the eyes delimited by well-defined dark medial and lateral bands. Upperparts greyish with a not well-defined middorsal broad blackish band from the neck to the base of the tail. Outside of limbs greyish. Chin, throat, lateral part of neck, inner side of upper arm and thigh buffy-orange, inner side of forearm and lower leg greyish-brown (extension of buffy-orange on the lateral part of neck wider than in A. nancymae). Upper side of tai I blackish , lower side reddish-orange.
3. General information
A. miconax belongs to the "red-neck group". It is endemic to Peru and inhabits the clouded forests of eastern Central Peru above 800 m a.s.l., sympatrically with Lagothrix flavicauda and Callicebus oenanthe. Nothing is known about its ecology, behaviour or social organization.
4. Status
This species is known only from skins and skulls collected in 1922 by E. Heller on the Rio Chinchao (Department of Huánuco), kept at the Field Museum of Natural History de Chicago, and in 1948 near the Rio Cayumba (Department of Huánuco), kept at the Museo de Historia Natural Javier Prado of the Universidad Nacional Mayor de San Marcos in Lima. These specimens were considered as A. trivirgatus until 1983, but reexamination by HERSHKOVITZ (1983) demonstrated that they belong to a new species. Due to the lack of information, its status is considered INDETERMINATE.
5. Distribution, conservation and protection (Map 6)
Taking the collecting localities as reference points, the hypothetical distribution corresponds to the cloud forests of eastern Central Peru, from the left bank of the Rio Huallaga in the Department of Huánuco) northward into the Department of Amazonas. This species is probably represented in the National Park Rio Abiseo.
G.) Aotus azarae Humboldt, 1811
1. Common name; local names Night monkey; Musmuqui, mono lechuza.
2. External characters
Similar to A. nigriceps and A. miconax but dense and velvety Pelage with much longer hair. Upperparts, sides and outside of limbs greyish. Throat, lateral part of neck, underparts and inner side of limbs brightly orange. Extension of this colour on the lateral part of neck above ventral half. Proximal portion of tail brightly orange, distal portion blackish.
3. General information
A. azarae belongs to the "red-neck group". Observations by RATHBUN & GACHÉ (1980) in Argentina, WRIGHT (1985) in Paraguay, and GARCÍA & BRAZA (1987,1993) in Bolivia show a similarity to A. nancymae and A. nigriceps with regard to habitat preference, diet, locomotion, social organization, use of sleeping sites and other ecological and behavioural aspects.
4. Status
The only available references for the presence of A. azarae in Peru arc the skins collected by E. Heller in October 1951 and January 1952 in the Province of Sandia (Department of Puno), which are deposited at the Field Museum of Natural History in Chicago. The status is considered as INDETERMINATE.
5. Distribution, conservation and protection (Map 6)
Taking the collecting localities as reference points and following the map provided by HERSHKOVITZ (1983), A. azarae is distributed in southeastern Peru, to the south of the rivers Madre de Dios and Inambari, in the Departments of Madre de Dios and Puno, respectively. This species is not represented in a protected area.
H.) Callicebus oenanthe Thomas, 1907
Common name; local names
Titi monkey; Tocón colorado, tití.
2. External characters
Pelage generally agouti-brown with fine, dense hair which is much longer than in other species of the genus. Crown with short greyish-brown hair, forehead generally with a white patch, face bordered by long buffy or whitish hair. Upperparts from the neck to the lumbar region and outside of arms predominantly agouti. Lumbar region and legs reddish-brown. Chin, underparts and inner sides of limbs reddish-orange. Tail brown-agouti. Further information is provided by HERSHKOVITZ (1988,1990).
3. General information
Until the last taxonomic revision of the genus, this species was considered as C. moloch. Based on phenotypical characters and the different type of habitat that it occupies, HERSHKOVITZ (1988, 1990) considered it as a distinct species. It is the smallest species of Callicebus in Peru. This endemic species lives in cloud forests above 800 in a.s.l., sympatrically with Aotus miconax and Lago1hrix.flavicauda. No information is available about its ecology or behaviour.
4.,Status
The status of the species is considered as INDETERMINATE.
5. Distribution, conservation and protection (Map 7)
According to HERSHKOVITZ (1988, 1990) this species is distributed in the cloud forests of northern Peru, in the Department of San Martín and probably also in the southern part of the Department of Amazonas, although the only records come from the valley of the upper Rio Mayo. It is not represented in any of the protected areas.
I.) Callicebus cupreus Spix, 1823
1. Common name; local names
Dusky titi monkey; Tocón, tocón colorado, tití.
2. External characters
Pelage generally greyish-brown. Forehead and crown dark-brown, reddish or grey.
Upperparts, upper arm and thigh greyish-brown, forearm and lower leg reddish-brown
to light-reddish. Underparts and inner sides of limbs varying from reddish-brown
and reddish-orange to buffy. Tail grey or blackish, terminal portion hoary.
The subspecies C. c. discolor has a wide transverse band of short, creamy-white
hair on the forehead; in C. c. cupreus the forehead is dark-brown.
3. General information
With a total length up to 85 cm and a weight of 1200-1500 g, C. cupreus belongs to the small,primates. It lives preferentially in bajiales of primary and remnant forests and in riverine forest. Activities take place in the lower and middle layers of the forest. Frequently descend to the ground to escape from avian predators. Groups comprise 2-5 individuals. Births occur seasonally between November and March. The single infant is carried and cared for by the father. The diet consists basically of fruits and insects, but flowers and tender leaves are also consumed.
C. cupreus lives sympatrically with C. torquatus. Their loud calls maybe easily confused by the unfamiliar observer, although clear sonagraphic differences exist. Loud calls of the former are soft and high-pitched, while those of the latter are low-toned and strong, and thus are audible at a major distances. Further information about ecology and behaviour are provided by KINZEY (1978), KINZEY & GENTRY (1979).
4. Status
Censuses by NEVILLE (1976) and FREESE et al. (1982) indicate their relative abundance in the majority of river basins. The species is hunted around large settlements. Its status is considered as COMMON.
5. Distribution, conservation and protection (Map 7)
C. cupreus is represented in Peru by two subspecies, C. c. discolor and C. c. cupreus. The former is distributed from the left bank the Rio Marañón to the Ecuadorian border and from the Rio Napo in the east to the Rio Chinchipe in the west. South of the Marañón it is found between the rivers Ucayali and Huallaga to the Rio Tambo.
C. c. cupreus is distributed from the Amazon river in the north to the Rio Purus in the Department of Ucayali in the south, and from the Rio Ucayali in the west to the Brazilian border in the east.
C. c. discolor is represented in the Pacaya-Samiria National Reserve and in the National Park of Tingo María, C. c. cupreus is fund in the Comunal Reserve Tamshiyacu-Tahuayo.
J.) Callicebus brunneus Wagner, 1842
1. Common name; local names Titi monkey; Tocón colorado, titi.
2. External characters
No detailed description can be given since we have no museum specimens available. During field observations we noted that forehead, forearms, feet and tail are blackish, upperparts and sides brown-agouti, underparts brownish or reddish-brown.
3. General information
Size and weight are similar to C. cupreus. This species lives preferentially in riverine vegetation and in pacales (associations of Guadua spp., Gramineae) of primary and remnant,forests. Travelling, foraging, and resting take place in the lower and middle layers of the forest. It sleeps in emergent trees provided with tangles of vines. Groups comprise 2-5 individuals. The birth season is not well-defined, although the majority of births is observed between October and February, coinciding with the period of major fruit availability. One infant is born per birth. The species is frequently encountered in interspecific association with S. fuscicollis weddelli and S. imperator. The diet consists basically of fruits and leaves and small quantities of insects and nectar. Further information is provided by CRANDLEMIRE-SACCO (1988), TERBORGH (1985), and WRIGHT (1985).
4. Status
Until 1988 this species was considered a subspecies of C. moloch, but in a taxonomic revision of the genus, HERSHKOVITZ (1988) described it as a distinct species. C. brunneus has been recorded from the Manú National Park (JANSON & TERBORGH, 1985) and from the Rio Tahuamanú (CASTRO et al., 1990; ENCARNACIÓN & CASTRO, 1990), where it seems to be fairly abundant. For other parts of its distributional range, information is lacking. Therefore, its status is considered INDETERMINATE.
5. Distribution, conservation and protection (Map 7)
According to HERSHKOVITZ (1988), this species is distributed in southeastern Peru, from the Rio Alto Purús in the Department of Ucayali south to the Bolivian border. It is represented in the Manú National Park.
K.) Callicebus caligatus Wagner, 1842
1. Common name; local names Titi monkey; Tocón colorado, tití.
2. External characters (Fig. 9)
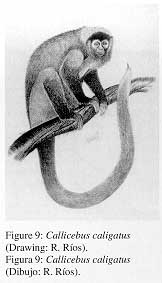 |
Pelage similar to C. cupreus, although the coloration is more greyish-brown. The most obvious phenotypic difference is the dark-brown colouration of crown and limbs. |
3. General information
No information is available about its ecology and behaviour.
HERSHKOVITZ (1988) considers this species as distinct from but sympatric with
C. cupreus cupreus.
4. Status
Due to the lack of studies and evaluations, the status is considered as INDETERMINATE.
5. Distribution, conservation and protection (Map 7)
According to HERSHKOVITZ (1988) C. caligatus is distributed east of the Rio Ucayali, from the Amazon in the north to approximately the Rio Tapiche in the south. However, during our explorations in this area we have not encountered this species.
L.) Callicebus torquatus Hoffmannsegg, 1807
1. Common name; local names
Yellow-handed titi monkey; Tocón, tocón,negro, tití.
2. External characters (Fig. 10)
 |
Pelage generally dark-brown. Few whitish hairs around mouth; forehead and cheeks blackish, crown dark-brown. Neck and upperparts dark brown, throat with a creamy-yellow patch. Upper arms and thighs dark-brown, forearms and lower legs blackish, hands with short creamy-yellow hair. Tail entirely dark-brown. |
3. General information
With a total length up to 97 cm and a weight of 1200-1500
g, C. torquatus belongs to the small primates. It lives exclusively in
primary forests of the varillal type. Travelling, foraging, and resting
take place in the lower and middle layers of the forest. It rarely descends
to the ground to catch insects or to escape from avian predators. Groups are
formed by an adult pair and its offspring of varying age and comprise between
2-5 individuals. Births occur seasonally between November and March. The single
infant is carried and cared for by the adult male. The diet consists basically
of fruits and insects, but flowers and tender leaves are also consumed. Further
information is provided by EASLEY (1982), EASLEY & KINZEY (1986), KINZEY
(1976, 1977, 1978, 1981), KINZEY & GENTRY (1979), KINZEY & ROBINSON
(1983), and KINZEY et al. (1977).
All species of Callicebus generally emit loud calls
in the early morning before leaving their sleeping site. This makes them easily
fall prey to human hunters.
4. Status
The attractively -coloured C. torquatus is one of the species that are difficult to observe in its natural habitat. The high hunting pressure drastically affects its populations. Fifteen years ago, this species could be observed frequently in the varillales of the rivers Itaya and Nanay, but now they are rarely observed or heard in these areas. Rapid deforestation along the Itaya and Nanay, accelerated by the construction of the Iquitos-Nauta highway, also contributes to the reduction of its populations. In conclusion, the species must be considered as VULNERABLE.
5. Distribution, conservation and protection (Map 7)
According to HERSHKOVITZ (1988) it is distributed in northeastern Peru north,of the rivers
Nanay and Amazonas to the Rio Putumayo. It is not represented in any of the protected areas.
M.) Pithecia monachus E. Geoffroy, 1812.
1. Common name; local names
Red-bearded saki; Huapo, huapo negro.
2. External characters (Fig. 11)
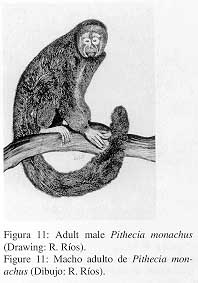 |
Pelage generally blackish with rough, long and slightly dense hair. Crown with short, stiff, brown-agouti hair. Upperparts and limbs dark-brown, hairs on upperparts and sides of the trunk with reddish-orange terminal band. Chest sparsely haired reddish-brown, belly dark-brown. Long blackish hair of tail with yellowish terminal band. |
3. General information
With a total length of 95 cm and a weight of 3 kg, this species
belongs to the medium-sized primates. It lives exclusively in primary forests
of both the bosque de altura and bajial type.
Travelling, foraging and resting take place in the middle and upper layers of the forest. Occasionally it descends to the ground to escape from avian predators. Groups formed by an adult pair and their offspring of different ages comprise 2-8 individuals. The birth season comprises the period between September and December. The single infant is carried and cared for by the mother. The interbirth interval is 2-3 years. The species is frequently encountered in interspecific association with Saguinus mystax, Saguinus fuscicollis and Cacajao calvus ucayalii. The diet consists of fruit pulp and seeds and small amounts of leaves and flowers, but some authors mention the consumption of small birds, rodents and insects. Further information is provided by BARTECKI & HEYMANN (1987), BUCHANAN et al. (1981), HAPPEL (1982), HEYMANN & BARTECKI (1990), and SOIN1 (1986).
4. Status
Censuses carried out south of the river Marañón and Amazonas and between the rivers Napo and Pastaza indicate that the species is relatively common. It is hunted for subsistence, to obtain infants as pets, and to use the tail and the teeth in rural handicraft. In forests remote from settlements the species can still be observed with certain frequency. It is common in the principal tributaries of the rivers Napo, Tigre and Marañón and in the Pacaya-Samiria National Reserve. The species is considered as COMMON.
5. Distribution, conservation and protection (Map 8)
The species is distributed from the Rio Putumayo in the north to about the Rio Alto Pur6s in the south. It is also found in the lower parts (between 700 and 800 m a.s.l.) of the Departments of Amazonas, Huánuco and Pasco. Between the rivers Napo and Tigre it lives sympatrically with P aequatorialis. Therefore, in faunal inventories of northeastern Peru where the species P monachus is quoted, it might well be P aequatorialis. A more detailed comparison of the external characters is recommended to adequately define the distributional limits.
The species is represented in Peru by the subspecies P m. monachus which is found in the National Park of Tingo María, Pacaya-Samiria National Reserve, and in the Comunal Reserve Tamshiyacu-Tahuyao.
N.) Pithecia aequatorialis Hershkovitz, 1987
1. Common name; local names Saki monkey; Huapo, huapo negro.
2. External characters (Fig. 12)
 |
Pelage generally greyish-brown with rustic, long and dense hair. Hair banded creamy-yellow subterminally. Crown densely covered with creamy-yellow to brownish hair. Back, sides and limbs greyish-brown except creamy-yellow hands and feet. Underparts from chin to belly sparsely covered with reddish hair. Tail with long greyish-black hair. Conspicuous sexual dimorphism: in the male the plushy crown has short, stiff, creamy-white hair; in the female the hair is longer, flowing and brownish with a subterminal creamy-white band. Further information is provided by HERSHKOVITZ (1987a). |
3. General information
The little existing information indicates that this species
is similar to Pithecia monachus in
body dimensions, group size and social organization. Further
information is provided by
BUCHANAN et al. (1981).
4. Status
Until 1979 two species of the genus Pithecia were considered for Peru: P monachus and P hirsuta (HERSHKOVITZ, 1979b). In a taxonomic revision of the genus, HERSHKOVITZ (I 987a) quoted the existence of P. aequatorialis and P irrorata, but suppressed the species name P hirsuta. P aequatorialis now represents the species that was previously considered as P monachus. Censuses and observations in the principal river basins in its distributional range indicate that this species is uncommon due to high hunting pressure. However, the sympatry between P monachus and P aequatorialis has not allowed the definition of its actual status; therefore, it is considered INDETERMINATE.
5. Distribution, conservation and protection (Map 8)
The distribution of P. aequatorialis is not yet well-defined. According to HERSHKOVITZ (1987a) it is found in a restricted area of northeastern Peru, on the left bank of the Rio Marañón between the rivers Tigre and Napo. Given the synipatry with R monachus it is recommended that studies of external characters and behaviour be intensified to clarify the distributional limits of both species. P aequatorialis belongs to the species that are not represented in a protected area.
0.) Pithecia irrorata Gray, 1844
1. Common name; local names Saki monkey; Huapo, huapo negro.
2. External characters (Fig. 13)
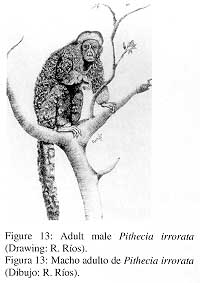 |
Pelage generally greyish with long, dense and shaggy hair. Hair with a creamy-yellow subterminal band. Differs from the other species of the genus in the crown which is densely covered with long hair (with a whitish terminal band), forming a tuft that is oriented towards the forehead. Back and limbs greyish, except hands and feet which are whitish. Whitish hair on the chest with a brownish band subterminally, hair on the belly completely brownish. Tail darkchestnunt, long hair with a creamy band subterminaily. Males differ from females by the lack of a moustache. Further information is provided by HERSHKOVITZ (I 987a). |
3. General information
With body dimensions similar to the other species of the
genus, it belongs to the medium-sized primates. It lives exclusively in primary
forests of southeastern Peru. For travelling, foraging, and resting, all forest
layers and emergent trees are used but the medium and upper layers are preferred.
It sleeps at the base of strong branches in leafy trees that are densely covered
with lianas. Like the other species in the genus it is very agile in its locomotion.
It responds to strange noises with alarm calls and escapes rapidly with leaps
of 5 meters or more. Groups formed by the adult pair and its offspring comprise
2-6 individuals. The birth season is not well-defined, but the newborn infants
are most frequently observed between September and January. The single infant
is carried and cared for by the mother. No detailed information about its ecology
and behaviour is available.
4. Status
This species was previously considered as P. hirsuta (HERSHKOVITZ, 1979), but since 1987 as a distinct species (HERSHKOVITZ, 1987a). The only records are from the Manú
National Park and from the rivers Tahuamanú and Acre in the Department of Madre de Dios. Censuses in the Manú National Park indicate a moderate abundance, but populations densities are much lower on the rivers Tahuamanú and Acre, mainly due to hunting for meat and to obtain body parts for handicraft. In the latter areas, the habitat is drastically reduced due to agricultural colonization. Further explorations in its distributional range are necessary to determine its status. For the moment it is considered as INDETERMINATE.
5. Distribution, conservation and protection (Map 8)
R irrorata is found from the Rio Alto Purus in the Department of Ucayali south to the rivers Madre de Dios and Inambari in the Department of Madre de Dios, and in parts of the Departments of Junín and Cusco (HERSHKOVITZ, 1987a). It is represented in Peru by the subspecies P. i. irrorata which is found in the Manú National Park.
P.) Cacajao calvus I. Geoffroy, 1847
1. Common name; local names
Red uacari (uakari); Huapo colorado, huapo rojo, mono inglés.
2. External characters (Plate 3)
 |
Pelage generally reddish with rustic, long and sparse hair. Face and forehead naked and pigmented brightly red. Crown with short reddish hair. Back and limbs covered with long reddish hair. Underparts reddish and thinly haired. Short tapered tail with moderately long hair. Males differ from females by the presence of bulging muscles on the temples, which give the appearance of a "squared face". Further information is provided by HERSHKOVITZ (1987b). |
3. General information
This is a moderate to large-sized species, with a body length
of up to 80 cm and a weight of 4-5 kg. It lives in primary forests of the bosque
de colina type but during fruiting periods it is frequently found in bajiales
and on restingas with associations of palms (palmales). Travelling,
foraging and resting take place in the middle and upper levels of the forest
and in the crowns of emergent trees. Uacaris are nearly always found in interspecific
association with other cebid monkeys (Saimiri sp., Cebus apella or Lagothrix
lagotricha). Troops contain 30-70 individuals, but R. Aquino has observed
a troop with more than 100 individuals on the Rio Orosa in May 1982. In
every troop, several adult males are present who lead the group during their
daily activities. During feeding and foraging, troops may split up into smaller
subgroups. The troop sleeps dispersed over two or more neighbouring trees. The
birth season is not well-defined, but the majority of births occur between December
and March. The mother carries and cares for the single infant. The diet consists
basically of fruits with seeds frequently being the part consumed; Mauritia
flexuosa (Palmae; local name aguaje), Couma macrocarpa (Apocynaceae;
local,name leche huayo) and Parahancornia sp. (Apocynaceae;
local name naranjo podrido) are among the fruits that are consumed. Flowers,
leaves, epiphytes and insect larvae have also been reported as part of their
diet. Further information is provided by AQUINO (1988), BARTECKI & HEYMANN
(I 987c), FONTAINE (198 1), and HEYMANN (I 989,1990d).
4. Status
Census work and explorations carried out by AQUINO (1988) indicate that this species has disappeared from many areas in northeastern Peru while in other part of its range populations have been diminished due to strong hunting pressure and habitat destruction. Its current status is considered as ENDANGERED.
5. Distribution, conservation and protection (Map 9)
In Peru, this species is represented by the subspecies C.
c. ucayalii. Its distribution is limited by the rivers Ucayali to the west,
Amazonas to the north, Yavarí to the east, and the Urubamba to the south.
During recent explorations it was not encountered in the basin of the Rio Urubamba,
and we suspect that it has been extinguished in this area.
The Comunal Reserve Tamshiyacu-Tahuayo is the only protected area that includes populations of C. c. ucayalii.
Q.) Cebus albifrons Humboldt, 1812
1. Common name; local names White-fronted capuchin; Machín blanco, mono blanco, martín.
2. External characters (Plate 4)
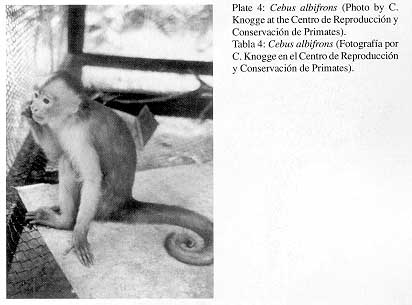 |
Pelage generally bright brown to yellowish-brown with fine, moderately long and dense hair. Crown with dark-brown short hair; face and forehead with sparse pale brown hair. Back and outside of upper arm and thigh light-brown, forearm and lower leg pale-brown. Underparts and inner side of limbs very light-brown. Semi-prehensile tail with moderately long light-brown hair. Further information is provided by HERSHKOVITZ (1949). |
3. General information
With a total length up to 100 cm and a weight of 2-3 kg,
this species belongs to the medium-sized primates. It lives exclusively in primary
forests of both the inundable and bosque de colina type. For travelling,
foraging and resting the medium and upper layers of the forest are used, but
occasionally these monkeys descend to the ground to collect fruits or to escape
from avian predators. It sleeps in the highest trees on branches covered by
lianas or between the fronds of palms like Mauritia flexuosa (Palmae;
local name aguaje) and Scheelea sp. (Palmae; local name shapaja).
Groups include 5-25 individuals and are polygamous, with several adult males
and females and their offspring. In northeastern Peru the birth season comprises
the period between September and March. The single infant is carried and cared
for by the mother. The diet consists basically of fruits and insect larvae,
but flower, leaves, bird eggs and small vertebrates are also consumed. Further
information is provided by FREESE (1977), FREESE & OPPENHEIMER (1981), MCVAY
(1983), VAN SCHAIK & VAN NOORDWIJK (1989), SOINI,(1986), and TERBORGH
(1983, 1985).
4. Status
C. albifrons, like the congenerous C. apella, is subject to a strong hunting pressure and has been extinguished in the forests around large human settlements. However, in some river basins like that of the Alto Tapiche, Curaray, Yavarí, Urubamba, Madre de Dios and others, it is still found in large numbers. Population densities are high in the Pacaya-Samiria National Reserve and in the Manú National Park. Its status is considered as VULNERABLE.
5. Distribution, conservation and protection (Map 10)
According to HERSHKOVITZ (1949) and GRIMWOOD (1968) the distributional
range comprises the whole selva baja (lowland rain forest) and part of
the selva alta (montane rain forest) up to about 1800 m a.s.l.. It is
also found on the Pacific forest in the north of the Department of Tumbes. The
species is represented in Peru by three subspecies (HERSHKOVITZ, personal communication)
whose distributional ranges are not yet well defined. The map provided here
has been elaborated on the basis of collecting localities of specimen deposited
at the Field Museum of Natural History in Chicago. C. a. yuracus is distributed
from the Colombian and Ecuadorian border in the north to the Rio Pachitea in
the south, and from the rivers Amazon and Ucayali in the cast to the Andean
foothills in the west. C. a. unicolor is found east of the Rio Ucayali
from the Amazon in the north to the Rio Alto Pur6s in the south. C. a. cuscinus
is distributed in southeastern Peru from the Rio Alto Pur-6s to the Bolivian
border and in the lower parts of the Departments of Junín and Cusco.
Specimens from the Departments of Huánuco and San Martín exhibit
phenotypical characters that are distinct from the other subspecies and might
probably represent a fourth subspecies. To verify or reject this assumption
further studies not only of skins and skeletons but also of ecological and behavioural
aspects are required. The subspecies living in the Pacific forests near Tumbes
has not yet been identified.
C. albifrons is represented in the National Parks of Manú and Yanachaga-Chemillén, in the Pacaya-Samiria National Reserve, and in the Comunal Reserve Tamshiyacu-Tahuayo. During our censuses and explorations we have noted that locally C. albifrons is found where C. apella is lacking and vice versa. We recommend that during future faunal inventories this observation be taken into consideration to determine the distribution and habitats of both species.
R.) Cebus apella Linnaeus, 1758
1. Common name; local names
Tufted capuchin, black-capped capuchin; Machín negro, mono negro, martín.
2. External characters
Pelage generally dark-chestnut to blackish-chestnut with slender, moderately long and not very dense hair. Crown with short blackish hair forming a cap that extends from the forehead to the back of the head. Crescent- shaped, creamy spot above each eye. Back, underparts, upper arms and thigh light-chestnut, forearms and lower legs dark-chestnut. Dark-brown band on the midline of the back from the neck to the base of the tail. Semiprehensile tail with moderately long, dark-chestnut hair. Further information is provided by HERSHKOVITZ (1949).
3. General information
With a total length up to 110 cm and,a weight up to 4 kg, this species belongs to the medium-sized primates. It lives in primary forests, both of the inundable and bosque de colina type. For travelling, foraging, and resting all layers of the forest are used, but the,lower and middle layers are preferred. It occasionally comes down to the ground to search for fruits and to escape from avian predators. It sleeps at the base of strong branches in tall trees with abundant lianas. Groups comprise 5-15 individuals and are polygamous, with several adult males and females and their offspring. The birth season is not well-defined but most births seem to occur between October and June. The single infant is carried and cared for by the mother. Its omnivorous diet is composed of fruits, tender leaves, insects and other invertebrates, bird and small mammals. During periods of fruit scarcity insects form the base of the diet, and hence groups are more widely dispersed at this time. C apella is nearly always found in interspecific association with S. sciureus or S. boliviensis. Further information about ecology, behaviour and social organization are provided by FREESE & OPPENHEIMER (1981), JANSON (1984, 1985a, 1985b, 1988, 1990a. 1990b), MCVAY (1983), PODOLSKY (1990), VAN SCHAIK & VAN NOORDWIJK (1989), SOINI (1986), and TERBORGH (1983,1985).
4. Status
Due to its size, C. apella is subject to a strong hunting pressure which has led to its extinction in areas around human settlements. Only in forests with little or no human interference, like the Pacaya-Samiria National Reserve and the Manú National Park, is it relatively abundant. Its status is considered as VULNERABLE.
5. Distribution, conservation and protection (Map 11)
According to HERSHKOVITZ (1949) and GRIMWOOD (1968) the distributional
range comprises the whole selva baja (lowland rain forest) and part of
the selva alta (montane rain forest) up to about 1800 m a.s.l.. The species
is represented in Peru by four subspecies (HERSHKOVITZ, personal communication)
whose distributional ranges are not yet well defined. C. a. maranonis is
distributed from the Colombian and Ecuadorian border in the north to about
the Rio Pachitea in the south, and from the rivers Amazon and Ucayali in the
east to the Andean foothills in the west. C. a. macrocephalus lives east
of the Rio Ucayali from the Amazon in the north to the Rio Alto Purus in the
south. C a. peruanus is found from the Rio Alto Purús south to
the rivers Madre de Dios and Inambari. C. a. pallidus is distributed
south of the rivers Madre de Dios and Inambari in the Department of Puno. The
specimens from the montane forests between 800 and 1000 m a.s.l. from the Departments
of Huánuco, Pasco and Junín show distinct phenotypical characters
and might belong to a fifth subspecies. To verify or reject this assumption
more detailed comparison of specimens and ecological studies are necessary.
C. apella is represented in the National Parks Manú and Tingo María, in the Pacaya-Samiria National Reserve, and in the Comunal Reserve Tamshiyacu-Tahuayo.
S.) Alouatta palliata Gray, 1849
1. Common,name; local names Mantled howler monkey; Mono coto de Tumbes, coto mono.
2. External characters (Fig. 14)
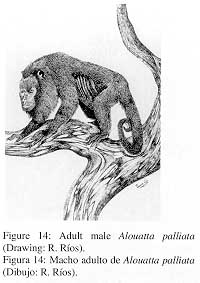 |
Pelage generally dark-brown, with rough and moderately long hair. The hair of the forehead is short and oriented backwards up to the crown where much longer hair forms a transverse crest. Cheek anteriorly with short and sparse dark-greyish hair, posteriorly with longer hair that contrasts with the fur of the rest of the head and forms a beard. Mantle and outside of limbs dark-brown with much shorter hair than in A. seniculus. Prehensile tail dark brown above, naked and with dermatoglyphs below. |
3. General information
A. palliata belongs to the large-sized primates. The
little information available from Peru,indicates that it lives preferentially
in humid semidense forests of the Pacific coast that are characterized by the
presence of dispersed trees of up to 20 m height and a predominance of bushy
vegetation. Groups comprise up to 11 individuals. It is folivorous like A. seniculus
but also includes fruits and flowers in its diet. Further information about
this species is provided by ESTRADA (1984), ESTRADA & COATES-ESTRADA (1984,
1985), GLANDER (1978, 198 1), MILTON (1980), and NEVILLE et al. (1988).
4. Status
Results of censuses by PULIDO & YOCKTEN (1983) indicate a sparse population. Its status is therefore considered ENDANGERED.
5. Distribution, conservation and protection (Map 12)
This species is represented in Peru by the subspecies A. p. aequatorialis, restricted to the National Forest of Tumbes on the border with Ecuador. During the last years the tropical forest of the Pacific coast has not only suffered from deforestation but also from serious alterations due to climatic,change, with negative effects upon the reduced population of these primates. To assure the integrity of,the forest would be an adequate means to protect this species.
T.) Aloualta seniculus Linnaeus, 1766
1. Common name; local names
Red howler monkey; Coto, coto mono, mono aullador.
2. External Characters (Fig. 15)
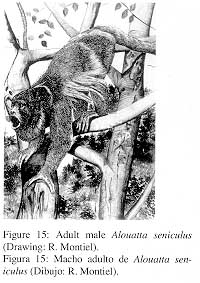 |
Pelage generally chestnut-red with rough, short and sparse hair. Face nearly naked and black. Crown with short, dark-chestnut hair. Throat and chin with longer hair. Upperparts, sides and limbs dark-chestnut. Prehensile tail on the upper side covered with short chestnut-red hair, lower side naked with dermatoglyphs. In the male, the hyoid bone is very large and serves as a sounding board for the deep vocalizations that are audible for a distance of 500 m or more. |
3. General information
With a total length of up to 100 cm and a weight of 5-7 kg,
this species belongs to the large primates. It lives exclusively in primary
forest both of the inundable and bosque de colina type. For travelling,
foraging and resting, all layers of the forest are used, but red howler monkeys
mainly stay in the upper levels and in emergent trees. They may descend to ground
to cross openings in the forest and to drink water from rivers and cañons.
Red howler monkeys are excellent swimmers and easily cross rivers. They sleep
in the most densely leaved trees with abundant lianas. Groups comprise 3-10
individuals and may include 1-3 adult males. The dominant adult male mates polygamously.
In northeastern Peru births are observed throughout the year but with major
frequency between October and April. The single infant is carried and cared
for by the mother. Red howler monkeys are folivorous and mainly consume mature
and tender leaves, petioles, buds and flowers, and a minor quantity of fruits.
Further information about ecology and behaviour is provided by BARBOSA (1988),
BRAZA et al. (1981, 1983), CUERVO DÍAZ (1986), GAULIN & GAULIN (1982),
NEVILLE (I 972a, 1972b), NEVILLE et al. (1988), SAAVEDRA (1985), SEKULIC (1982),
and SOINI (1986).
4. Status
The numerous censuses indicate reduced population densities as a consequence of the high hunting pressures to which this species is subject to like all the other large primate species.
Reduced populations are found in renacales (associations of Ficus spp., Moraceae) and aguajales de bajial (associations of Mauritia flexuosa, Palmae), which are practically inaccessible to man. The species is relatively abundant in the Pacaya-Samiria. National Reserve and in the Manú National Park. Its status is considered as VULNERABLE.
5. Distribution, conservation and protection (Map 12)
According to GRIMWOOD (1968), the distributional range comprises the whole lowland rainforest area. However, his information is outdated and populations have declined particularly in the lower parts of the selva alta as a result of human colonization and deforestation. For instance, in the valley of Chanchamayo (Department of Junín) the species was reported by GRIMWOOD (1968) to occur between 1000 and 1500 m a.s.l., but now it is no longer found there. This species is present in the Manú National Park, the Pacaya-Samiria National Reserve, and in the Comunal Reserve Tamshiyacu-Tahuayo.
U.) Lagothrix lagotricha Humboldt, 1812
1. Common name; local names Woolly monkey; Choro, mono barrigudo.
2. External characters (Fig. 16)
 |
Coat colour generally between intense auburn to dark-grey. Upperparts, arms and legs with moderately long hair, ventral parts with longer hair but less densely covered. Bare face black. Crown with short hair. Terminal portion of prehensile tail hairless and with dermatoglyphs. |
3. General information
Large primate, reaching up to 150 cm total length and 5-8
kg of weight. It lives in primary rain forests, of both the inundable and the
bosque de colina type. The upper levels and emergent trees of the forest
are used for travelling, foraging and resting. Rarely it descends to the ground
to escape from raptors (hawks, eagles, and falcons), especially the harpy eagle
Harpia harpyja. It prefers to sleep in most densely leaved trees on strong,
horizontal branches. Locomotion is rapid and very agile. Reacts to the presence
of man with noisy vocalizations and sudden branch-shaking. Groups comprise 15-60
members with adults, juveniles and infants of both sexes. One infant is born
per birth and is carried and cared for by the mother. The birth season is not
well-defined, but in northeastern Peru the majority of births occur between
September and April. The diet consists basically of fruits, but leaves, flowers,
insects and small vertebrates are also consumed. Further information is provided
by DEFLER (1989), KAVANAGH & DRESDALE (1975), NISHIMURA (1990), NISHIMURA
& IZAWA (1975), RAMÍREZ (1980, 1988), SOINI (1986, 1990), and STEVENSON
et al. (1994).
4. Status
Numerous censuses indicate that population densities are very low throughout most parts of the distributional range, which includes river basins where the species has been virtually extinguished. The decline of populations is due to a high hunting pressure and to deforestation. Its status is considered as ENDANGERED.
5. Distribution, conservation and protection (Map 13)
According to GRIMWOOD (1968) the distributional range includes the entire selva baja and parts of the selva alta up to 1800 m a.s.l.. L. lagotricha is represented in Peru by three subspecies (FOODEN, 1963) whose distribution is not yet well-defined: L. 1. lagotricha on the left bank of the rivers Napo and Amazon; L. 1. poeppigii north of the Marañón from the Napo in the east to the Cordillera Oriental in the west, and south of the rivers Marañón and Amazon on both sides of the Ucayali approximately up to the Rio Pachitea; L. l. cana in southeastern Peru from the Rio Alto Purus in the Department of Ucayali to the Bolivian border, and also in parts of the Departments of Pasco and Junín. The species is represented in the National Reserve of Pacaya-Samiria, in the Man6 National Park and in the Comunal Reserve Tamshiyacu-Tahuayo.
V.) Lagothrix flavicauda Thomas, 1927
1. Common name; local names Yellow-tailed woolly monkey; Barrigudo andino, choro cola amarilla, maquisapa chusco.
2. External characters (Fig. 17)
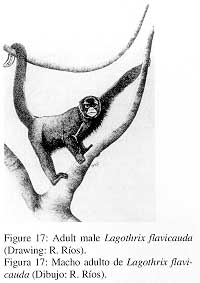 |
Size and weight similar to L. lagotricha, but looks more robust due to long, dense hair. Face black with a triangular area of white short hair covering nose and mouth. Ears densely covered with moderately long hair. Coat of head and upper part of back black with an auburn tone that increases towards the back until becoming a red-auburn on lower parts of back and on tail. Underparts with long, dark-brown hair. Short and robust arms and legs with short, dark-brown hair, hands and feet with moderately long hair on all digits. |
Underpart of prehensile tail with a broad golden-yellow band on distal half, terminal portion bare with dermatoglyphs. Scrotum covered with a tuft of long golden-yellow hair.
3. General information
The sparse information available about L. flavicauda indicates that it lives in the montane rain forest and cloud forest between 1800 in a.s.l. and 3000 in a.s.l. where vegetation is composed of trees that reach heights of 20-40 m and have diameters of less than I meter. It lives in groups of 4-14 members including 1-3 adult males. The diet consists of fruits, flowers, roots of epiphytes, leaves, buds, and petioles. Further information is provided by GRAVES & O'NEILL (1980), LEO (I 980,1982a, 1982b, 1989), LEO & ORTÍZ (198 1), MACEDO-RUÍZ & MITTERMEIER (1979), and MITTERMEIER et al. (1977).
4. Status
L. flavicauda is considered to be in immediate danger of extinction (LEO & ORTÍZ, 1981; MITTERMEIER et al., 1977). Principal causes are habitat destruction and subsistence hunting. The construction of the carretera central, the central highway along the cloud forests of the Departments of San Martín and Amazonas, has lead to increased deforestation for agriculture and stock-farming by human immigrants from the Cajamarca region. L. flavicauda is considered as ENDANGERED.
5. Distribution, conservation and protection (Map 13)
The distribution comprises the western part of the Department of Amazonas and the eastern part of the Department of San Martín. Until May 1974, it was considered as extinct. Soon after its rediscovery, the National Park of Rio Abiseo was created to protect the yellow-tailed woolly monkey together with other mammals and the flora, of which many species are endemic to this region.
W.) Ateles belzebuth E. Geoffroy, 1806
1. Common name; local names
White-bellied spider monkey; Maquisapa, mono araña.
2. External characters (Fig. 18)
 |
Pelage with rough, little dense and long hair. Bare face dark-brown, except nose, surrounding of eyes and lips which are flesh-coloured. Tuft of long black hair on the head oriented towards the forehead, brow with transverse yellow-creamy band. Dorsal part of rump and external parts of arms with long black hair; ventral part of rump and inner side of arms with yellow-creamy hair; legs dark-orange. Prehensile tail black above, yellow-creamy below, terminal portion hairless below with dermatoglyphs. |
3. General information
Size and weight are similar to A. paniscus. It lives
in primary forests of both the inundable and the bosque de colina type.
The upper levels of the forest and emergent trees are used for locomotion, foraging,
and resting. It sleeps in the most densely leaved trees with abundant lianas
and epiphytes. Groups of up to 20 individuals are formed by adults, juveniles
and infants of both sexes. Generally, one infant is born per birth and is carried
and cared for by mother; no data are available about timing of birth during
the year. During periods of fruit scarcity groups split up into smaller foraging
units which regroup again in the evening to sleep together. It feeds on fruits,
flowers, tender leaves and small vertebrates. A. belzebuth and A. paniscus
are important seed dispersers. Further information about ecology and behaviour
is found in AHUMADA (1989), KLEIN (1972), and KLEIN & KLEIN (1976).
4. Status
A. belzebuth was not encountered during any of the censuses carried out between 1976 and 1987 which indicates that it is extinct in wide parts of its distributional range. Small remnant populations, subject to a strong hunting pressure, are known from the Rio Alto Curaray, an affluent of the Rio Napo. In conclusion, we consider this species as ENDANGERED.
5. Distribution, conservation and protection (Map 14)
According to GRIMWOOD (1968), this species is distributed in the northern parts of Peruvian Amazonia in the Departments of Loreto, San Martín and Amazonas. This little-known species is frequently considered as a subspecies of A. paniscus. It will be necessary to compare their external characters to define the limits of their distribution in Peru.
X.) Ateles paniscus Linnaeus, 1758
1. Common name; local names
Black spider monkey; Maquisapa, mono araña.
2. External characters (Fig. 19)
 |
Hair entirely black, moderately long and little dense. Bare face with slight black pigmentation except nose, surroundings of eyes and lips which are flesh-coloured. Tuft of long hairs on the head oriented towards forehead. Terminal portion of prehensile tail hairless below and with dermatoglyphs. |
3. General information
With a total length up to 150 cm and a weight up to 10 kg,
A. paniscus is the largest Peruvian primate species. It lives in primary
forests both of the inundable and bosque de colina type. The upper levels
of the forest and emergent trees are used for locomotion, foraging, and resting,
but occasionally it comes down to the ground to protect itself from avian predators.
Movements are very agile and the prehensile tail functions as a "fifth
hand". It lives in groups of 2-25 individuals that split up into small
subgroups during foraging and travelling, especially during periods of resource
scarcity. Contact between subgroups is maintained by means of vocalizations.
Groups rearrange in the evening to sleep together at the base of branches in
the highest and most densely leaved trees. One infant is born per birth and
is carried and cared for by the mother. The birth season is not well-defined,
but births are most frequently observed between October and April. The diet
consists basically of fruits, but leaves, flowers and small invertebrates are
also included. Further information about ecology and behaviour can be found
in DURHAM (1975), MCFARLAND (1987a, 1987b, 1988a, 1988b), VAN ROOSMALEN (1985),
and WHITE (1986).
4. Status
Second only to Lagothrix flavicauda, A. paniscus and Ateles belzebuth are the Peruvian primates that are most strongly affected by high hunting pressure and deforestation. Consequently, A. paniscus is considered as ENDANGERED. It can no longer be encountered in the surroundings of human settlements. Reduced populations have been found on the Rio Alto Yavarí and in the National Reserve of Pacaya-Samiria, while the Manú National Park is the only site where it still exists in relative abundance.
5. Distribution, conservation and protection (Map 14)
This species is represented in Peru by the subspecies A. p. chamek. According to GRIMWOOD (1960) and FREESE (1977), it is distributed south of the rivers Amazon and Marañón, from the Rio Huallaga in the west to the Rio Yavarí in the east. This species has not been recorded in the area between the rivers Marañón and Putumayo. In the southeast it is found in the Departments of Ucayali and Madre de Dios. Given its conservation status, it is necessary to establish a protected area between the rivers Yavarí Mirí and Alto Yavarí, where remnant populations of A. paniscus and also of C. ca1vus ucayalii are found.
